All you need to know about the world’s second malaria vaccine

Prof Adrian Hill, director of the Jenner Institute at the University of Oxford in England, during the interview at the Grand Challenges Annual Meeting in Dakar, Senegal.
What you need to know:
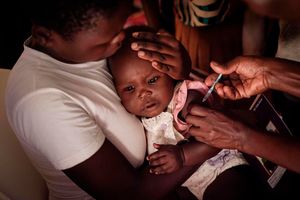
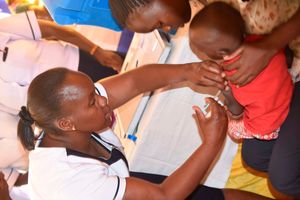
- Earlier this month, the World Health Organization (WHO) recommended a new vaccine — R21/Matrix-M — the world's second malaria vaccine, for the prevention of the deadly disease in children.
- This came after the phase III trials that enrolled 4,800 children aged between five months and three years in Mali, Burkina Faso, Tanzania and Kenya.
Earlier this month, the World Health Organization (WHO) recommended a new vaccine — R21/Matrix-M — the world's second malaria vaccine, for the prevention of the deadly disease in children.
This came after the phase III trials that enrolled 4,800 children aged between five months and three years in Mali, Burkina Faso, Tanzania and Kenya.
Last week at the Grand Challenges Annual Meeting (GCAM2023) in Dakar, Senegal, Healthy Nation caught up with Prof Adrian Hill, who played a key role in developing the R21 vaccine. He is the director of the Jenner Institute at the University of Oxford in England which focuses on designing and developing vaccines for infectious diseases prevalent in developing countries.
What vaccines are you working on and what does the recommendation of R21 by WHO mean?
We are working on R21, a vaccine for malaria. It has just been given a policy recommendation by WHO meaning that soon it could be rolled out in large amounts to protect particularly African children.
What makes R21 different from the world’s first malaria vaccine (RTS, S)?
It’s different in three way. Firstly, the vaccine is in abundant supply. Probably more than 100 million doses will be distributed next year.
Secondly, the cost for R21 will be much lower compared to other vaccines. We are looking at three to four dollars. The price will be announced soon, but we are expecting it to be lower. Furthermore, the vaccine is being manufactured in low doses, which explains why the price will be lower.
What is the efficacy of R21 vaccine?
This is a high efficacy vaccine, about 75 per cent on average. Apart from having that high efficacy in the first year, it also maintains it.
We recommend one booster dose at the moment and as it turns out, WHO is now recommending that the vaccine be used not only in high transmission areas but also in low to moderate transmission areas.
Are there any risk factors associated with the R21 vaccine?
As with all vaccines, some children can get fever, but compared to other vaccines, the risk profile of R21 is lower.
I was going through the findings from the study conducted on the R21 vaccine and I noticed that investigators highlighted cases of febrile convulsions/seizures. Would you explain why this happened?
Yes. Children can occasionally have a convulsions because they get fever after vaccination. This applies to most vaccines. But this is what we call self-limiting – the side effects do not last for long and the child recovers completely.
We are now seeing mosquitoes in places they never used to exist. What are you observing when it comes to new trends of infections?
Malaria will become widespread particularly because different species are moving to places they were not previously commonly found due to climate change. Most worrying is the arrival of Asian mosquitoes that like to live in cities. They do not necessarily bite at night, threatening the efficacy of bed-nets.
I think these new results with the R21 malaria vaccine are coming at a crucial time because we may need to distribute the vaccine much more widely than was the case five years ago.
At the Grand Challenges Annual Meeting this year, what is interesting and important in the fight against malaria?
This is the 20th Grand Challenge Annual Meeting. It is an exciting forum because there is always new technology emerging and it is applicable for malaria control.
We can now not only just protect children better than we ever could, but we also have a range of new tools that will help us eradicate this parasite.