Kenya to benefit from historic malaria vaccine funding
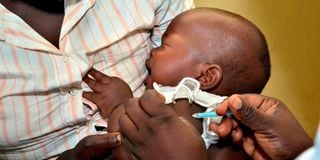
A baby receives her second dose of malaria vaccine at Ndhiwa Sub-County Hospital in Homa Bay County on April 16, 2021.
What you need to know:
- This international support of nearly Sh19 billion from 2022 to 2025 will facilitate increased vaccine access to children at high risk of illness and death from malaria.
- It will start with Ghana, Kenya and Malawi, the three African countries that began pilot introduction of the vaccine in 2019, and then expanding to other eligible endemic countries.
Kenya is among countries that will benefit from a landmark opportunity by Vaccine Alliance Gavi for nations to apply for billions of shillings in funding to introduce or further roll-out the RTS,S/AS01 (RTS,S) malaria vaccine.
This international support of nearly Sh19 billion from 2022 to 2025 will facilitate increased vaccine access to children at high risk of illness and death from malaria, starting with Ghana, Kenya and Malawi, the three African countries that began pilot introduction of the vaccine in 2019, and then expanding to other eligible endemic countries.
“Gavi’s new funding opportunity brings us one step closer to reaching millions more children across Africa with the lifesaving RTS,S malaria vaccine,” said Dr Matshidiso Moeti, WHO Regional Director for Africa, during a virtual press conference.
Following WHO’s recommendation in October 2021 for widespread use of the RTS,S among children in regions with moderate to high Plasmodium falciparum malaria transmission, a number of malaria-endemic countries have expressed interest in adopting the vaccine and are expected to apply for Gavi support to introduce the jab.
The RTS,S vaccine works specifically against Plasmodium falciparum, which is the deadliest malaria parasite and the most prevalent on the African continent. Where the vaccine has been introduced, there has been a substantial drop in children being hospitalised with severe malaria and a drop in child deaths in the age group that is eligible for the vaccine.
Gavi has indicated that the first application deadline in September 2022 is reserved for countries currently piloting the vaccine and for which continuity of the vaccine programme is a priority. A second window open to other eligible malaria-endemic countries will close in January 2023. Countries can already submit expressions of interest during the first funding window for inclusion in this round.
According to Thabani Maphosa, managing director of Country Programmes at Gavi, though initially supply will not meet demand, the vaccine alliance will look forward to work with countries and their partners to introduce and scale this new tool in the fight against malaria.
According to a WHO-commissioned global market study, over the next few years, the supply of the RTS,S malaria vaccine will be insufficient to meet the needs of over 25 million children born each year in areas where the vaccine is recommended. Should a second malaria vaccine complete clinical development successfully and be approved for use, the period of constrained supply could be shorter. The demand is estimated to range from 80 to 100 million doses annually.
In response to the supply situation, WHO has developed, with expert advice, a framework to guide vaccine allocation decisions at global and country levels that ensures children at highest risk across endemic countries are prioritised to receive the vaccine. The framework also aims to ensure that childhood vaccination services started in the three pilot countries continue without disruption, until supply fully meets demand.
According to Dr Moeti, now is the time for African countries and communities to call out their interest – to donors, health leaders and manufacturers – in early access to this vaccine. “This situation underlines once again why expanded local production of vaccines is essential for meeting health needs in Africa. We’ve seen encouraging first steps in that direction in recent months, and we are committed to supporting further efforts to expand vaccine production in Africa.”
WHO, Gavi and partners are working to accelerate RTS,S supply by exploring approaches to increase manufacturing capacity, market-shaping and facilitating the development of other first-generation and next-generation malaria vaccines.