Leaders rally behind injectable medication to free world from HIV
New technologies have resulted in long-acting injectables for HIV prevention and treatment in the last two years.
What you need to know:
- In Kenya, about 17,000 people were newly infected with HIV last year.
Global HIV leaders say there is a need for scaling up the use of long-acting options used in the treatment and prevention of HIV.
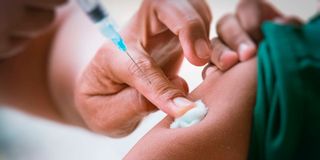
A long-acting HIV treatment and prevention tool is a medication that is injected, rather than a pill, and taken less frequently than daily. It can also be inserted, infused, or implanted in a person’s body from once a month to once a year to provide sustained protection from acquiring HIV or to suppress the virus.
The leaders, who are currently in Nairobi attending the 55th Programme Coordinating Board for the Joint United Nations Programme on HIV/AIDS (UNAIDS), say new technologies that have resulted in long-acting injectables for HIV prevention and treatment in the last two years should be embraced at scale, and equally, around the world.
Even though the world has existing HIV prevention tools and is still coming up with new technologies, UNAIDs data shows that about 1.3 million people were infected with HIV in 2023 alone.
Lower the number
In Kenya, about 17,000 people were newly infected with HIV last year. Scientists say that long-acting injections as prevention methods could lower this number compared to the oral methods that were available before.
In January 2021, the United States Food and Drug Administration (FDA) approved the first ever long-acting HIV antiretroviral therapy (ART) used as a treatment option.
The drug was to be given monthly as an injection. A year later, they approved a once-in-every-two months’ injection for the same drug.
The first long-acting injectable antiretroviral therapy (LA ART) known as CAB/RPV is a combination of cabotegravir and rilpivirine, which was already being used for HIV treatment but in a tablet form.
On March 31, 2022, Kenya’s first patient was injected with the drug in a study conducted by the Aga Khan University Hospital.
In December 2021, FDA approved the first ever long-acting drug used to prevent HIV.
The long-acting injectable cabotegravir (CAB - LA) was touted as a better option for pre-exposure prophylaxis (PrEP) used by at-risk populations to prevent HIV infections.
At the time, the only available option was an oral tablet taken daily but users found it hard to take the pills on a daily basis just for prevention.
As of now, there are about four types of long-acting antiretrovirals in use in some countries and many more are still being studied.
A study published in Lancet HIV in 2023 shows that early demonstration studies of injectable long-acting ART show high levels of adherence to injection schedules and continued viral suppression.
Winnie Byanyima, executive director of UNAIDS, said during the “Leadership in the AIDs Response” session that there needs to be technological innovation; insisting that the access has to be for everyone.
“Let us learn from the painful lessons of the past so that we write a new story now. In the late 1990s and early 2000s, even after antiretroviral medicines were proven to be effective and rolled out in high-income countries, 12 million people on this continent still died waiting for those drugs. We can - and must – do better with long-actings. We urge the companies producing these medicines to expand their generics licences. And we support governments making use of all their legal flexibilities to get access to affordable medicines,” she said.
Winnie lamented that countries from the Global South are usually the last to receive such life-saving drugs even when the burden for HIV is high in those regions.
“What if we do not wait for years, what if we ensure that science is treated as the public good it is? What if we disrupt the far too slow trajectory we are on and shift to a trajectory that accelerates progress, ends the pandemic, enables sustainability, and can be a model for the world?" She asked.
Dr Cissy Kityo, executive director of the Joint Clinical Research Centre, Uganda, a leading scientist working on trials of long-acting medicines, said the world has fantastic new tools with clear evidence on long acting medicine for both prevention and treatment.” Countries need to learn how well to use the drugs in order to prevent new infections and lower the viral load for people living with HIV.”
Africa’s Head of EVA Pharma, Dr Sylvia Vito, a company in Egypt licensed to produce a generic version of lenacapavir, said their company will not sit comfortably, instead, it will support the unmet HIV medical needs for those in need.
“We intend to move fast on product development, production, and eventual registration. It is our intention that high quality long-acting generic ARV medicine will not only be available, but made accessible and affordable as well. We intend to beat the current standard of care in HIV treatment and prevention by going further to improve on the current options for patients in low and middle-income countries.”
The drugs, however, remain expensive for people, especially those in the low- and middle-income countries.
In a press statement by UNAIDs, they mention that even though Gilead Sciences, the producer of lenacapavir, one of the new class of long-acting medicines, has not yet announced the price of its product for use as PrEP, it costs around $40,000 per person per year in the United States, where it is used for treatment.
“Experts have estimated that it could be produced and sold for $40 (about Sh5,180) per person per year, in line with UNAIDS estimates for sustainable pricing in low- and middle-income countries,” says the statement.