Shame of frequent recall of substandard and falsified drugs
Kenya’s porous borders with Uganda, Tanzania, Somalia, and Ethiopia allow counterfeit drugs to slip through unmanned routes. Some are hidden in trucks transporting legitimate goods.
What you need to know:
- In 2024, the drug regulatory authority made a total of 32 recalls. And in 2023, 18 health product recalls were reported. How did these medical products end up in the Kenyan market?
Scenario 1
As a rule of thumb, Esther Kinyua, a mother of two, buys medication from reputable pharmacies. According to Esther, she relies on certain visual and service-based cues to determine whether a pharmacy is reputable - bright, polished counters, uniformed attendants, and neatly arranged shelves stocked with familiar brands… the kind of pharmacies that print crisp receipts and have a pharmacist who nods reassuringly when handing over a prescription.
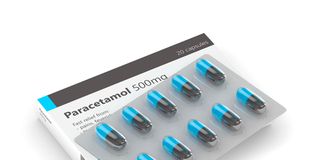
Paracetamol is one of the most common drugs in the country. It was recalled on January 22 last year.
It’s her way of being cautious and avoiding the horror stories she has heard too often– fake and substandard products in the market. Esther doesn’t need statistics to know they exist. Even without numbers, she has seen it on social media or encountered media reports.
What she doesn’t realise is just how deep the crisis goes. The World Health Organization estimates that at least one in 10 medicines in low- and middle-income countries are substandard or falsified. This means that for every 10 prescriptions filled, one could be counterfeit, ineffective at best, and deadly at worst. Yet, these faulty drugs continue to find their way onto the very shelves she trusts, slipping past regulations and into the hands of unsuspecting patients like her.
It’s worth pausing here to understand exactly why Esther—cautious as she is—finds herself at the centre of this story. In April last year, she was among the many parents voicing their alarm on social media after the Pharmacy and Poisons Board (PPB) recalled a widely used cough syrup. The news sent a chill down her spine. That very bottle had been sitting in her medicine cabinet. She had already given it to her baby—more than once—trusting it to soothe rather than harm.

In December 11, substandard sure lubricated condoms were recalled.
The reaction to that was, well, what you might expect: worry, fear and panic.
Scenario 2:
According to the National Syndemic Diseases Control Council, Kenya has the seventh-largest HIV burden globally, with approximately 1.4 million people living with HIV, and the sixth-highest number of HIV-related deaths. Given the scourge of HIV/Aids, many people use condoms as a protective measure. To some, condom use is a reliable method of contraception, preventing unintended pregnancies while offering protection against sexually transmitted infections.
But imagine walking into a shop, buying a condom, and using it—never realising that the thin layer of protection you relied on was faulty. Many never question what they purchase, trusting that a sealed packet guarantees safety. Yet, for some, that trust is shattered only when the consequences emerge—an unintended pregnancy, an STI, or, in the worst cases, a HIV diagnosis.
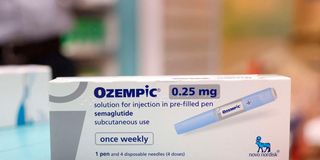
In July last year, the Pharmacy and Poisons Board warned that there were some pens falsely labeled as Ozempic that were sold to Kenyans.
“If they are made from substandard material, they could break or tear when in use,” says Purity Ireri, a nurse based in Embu.
Healthy Nation posed a question in a WhatsApp group, asking members if they ever thought twice about the quality of the condoms they used. The responses were telling—most admitted they never had. “It’s sealed, it’s sold in a shop, so it must be safe, right?” One person responded. It’s an assumption many make, trusting that what’s available on the shelves meets safety standards. But the reality is far more alarming.
There have been at least three instances where counterfeit condoms were found in circulation in Kenya, with the most recent case reported just last year.
Scenario 3:
Imagine this: You’re feeling unwell and visit a public health facility. After a diagnosis, the doctor prescribes medication, and you make your way to the pharmacy—right within the hospital compound, officially run by the facility.
You leave the pharmacy, medication in hand, certain that treatment is underway. But without your knowledge, the drugs you’ve been given are expired—no longer effective, and potentially harmful.
A recent audit report by Auditor-General Nancy Gathungu revealed a troubling reality. The report highlighted a health facility grappling with a severe drug shortage, where expired and unexpired medications were being mixed. This meant that patients were left to gamble with their health, uncertain whether they would walk away with expired or viable medication.
The report noted that Longisa County Referral Hospital in Bomet County had 85 units of expired drugs, including intraocular lens and Arteriovenous Fistula set — a set of medical equipment used for creating a connection between an artery and a vein, often created for dialysis purposes.
Even after the Auditor-General raised the red flag, the expired drugs remained at the facility for months. Bomet Governor Hillary Barchok admitted their presence, acknowledging the lack of a proper disposal plan. This oversight left patients vulnerable as the expired drugs stayed in the facility without being removed or safely discarded, posing a risk to public health, he acknowledged.
WHO explains that substandard products are those that do not meet quality thresholds because of poor manufacturing practices and subpar quality control.
All countries have their drug and health products watchdog, and Kenya’s is the Pharmacy and Poisons Board.
Although drug recalls make up an effective way to avoid possible risks associated with a marketed drug product, they also can lead to the disruption of inventories across the pharmaceutical supply chain, causing temporary or long-term shortages on the market.
Monitoring the safety and quality of pharmaceutical products in the market allows manufacturers to take necessary actions, such as recalls, if the risk of use of the product outweighs the benefit.
What is a drug recall?
In Kenya, it is the PPB that calls the tune when it comes to drug recalls. Drug recall is the official removal of some batches of medical products because of their quality, safety or efficacy, according to PPB’s regulatory standards.
Drugs could either be recalled voluntarily or they could be recalled by the drug regulatory body, especially when they are a threat to the public’s health.
In just one year -2024- , PPB made a total of 32 recalls. In 2023, the drug regulatory authority reported 18 health product recalls. “Medicines are recalled from the market if they are substandard or falsified. Most of the recalled medicines have been substandard,” it told Healthy Nation.
To understand how counterfeit drugs end up in the Kenyan market, the Healthy Nation turned to PPB for insight. According to the pharmacy board, authorised health products and technologies enter Kenya through various gazetted ports, including Jomo Kenyatta International Airport, Eldoret International Airport, Kilindini Port, and several land borders such as Busia, Namanga and Malaba.
“At these points, qualified regulatory officers, in collaboration with customs personnel, ensure 100 per cent verification of pharmaceutical consignments to guarantee they are registered and legitimate,” a PPB spokesperson explained.
However, despite these measures, falsified products still manage to infiltrate the market. “Smuggling remains a significant issue,” PPB noted. “Kenya’s porous borders with Uganda, Tanzania, Somalia, and Ethiopia allow counterfeit drugs to slip through unmanned routes. Some are hidden in trucks transporting legitimate goods.”
Illegal online pharmacies and unregistered websites also contribute to the problem. These platforms, often operating through social media pages, sell counterfeit medicines directly to unsuspecting consumers. “Some online sellers mix genuine and fake products, making it difficult for the average person to tell the difference,” the PPB representative added.
Another concerning method is the diversion and repackaging of legitimate drugs. “Unscrupulous distributors swap authentic products with counterfeits or repackage expired and substandard drugs with new labels before pushing them back into the supply chain,” the spokesperson explained
Finally, fraudulent importation permits also play a role. “Some criminals forge permits to import counterfeit medicines, and at times, even legitimate distributors unknowingly source products from unverified suppliers,” the PPB warned.
The drug regulatory authority said that it has not had any records of any side effects from patients who used the drugs that have been recalled. “PPB ensures the mop-up of potentially harmful drugs from the Kenyan market, primarily through post-market surveillance. This involves the continuous monitoring of drugs after they've been released, including the collection and analysis of poor-quality health products and technology reports from healthcare professionals, consumers, and manufacturers. When quality concerns arise, PPB issues drug recalls, ensuring public safety. This is supported by regular inspections of medicines manufacturing and distribution sites.
Healthy Nation analysed some of the recent recalls and got a toxicologist to explain their impacts.
2024 recalls:
JANUARY 22: Medicine Quality Alert: Class II Medicines Recall of Blink Injection (Paracetamol )
This is one of the most common drugs in the country. It was recalled on January 22 last year. With a pack size of about 100 millilitres and an expiry date within the year that it was recalled, PPB found that it had a colour change. It is supposed to be a clear colourless solution, but those that were recalled were yellow or pale amber.
Toxicology view: If a drugs shows change of colour, it is an indicator of contamination.
APRIL 11: PUBLIC ALERT ON BENYLIN PAEDIATRIC 1OOMLs COUGH SYRUP, BATCH NO. 329304 MANUFACTURED BY JOHNSON & JOHNSON (Pty), SOUTH AFRICA.
The drug was recalled because of the undesirable high levels of diethylene glycol, an ingredient that was detected after a laboratory analysis was done by the National Agency for Food and Drug Administration and Control of Nigeria.
Toxicology view: Diethylene glycol has been used as a solvent in liquid medicines. Excessive amounts will lead to various toxicities. It should never be used in pharmaceutical formulations due to its toxicity, which can cause severe kidney and neurological damage, and has led to numerous poisonings and deaths, especially in children.
MAY 11: Fake breast cancer drug
The PPB announced that there was a fake breast cancer drug called Herceptin (Trastuzumab 440g) circulating in the market.
Toxicology view:
Herceptin is a drug used in both stomach and breast cancer. The presence of substandard drug poses a risk of having various contaminants or reduced dosage posing a risk of treatment failure. JULY 18: PUBLIC ALERT: FALSIFIED OZEMPIC (SEMAGLUTIDE) PENS
In July last year, the Pharmacy and Poisons Board warned that there were some pens falsely labeled as Ozempic that were sold to Kenyans. Instead, another drug for diabetes called the Apidra Solostar Pens was the one behind the veil of the Ozempic label. PPB has not yet registered Ozempic for use in Kenya.
Toxicology view: Semaglutide is an anti-diabetic medication used for the treatment of type 2 diabetes and an anti-obesity medication used for long-term weight management.
Apidra is a fast (rapid) acting insulin used for adults and children with type 1 diabetes to control high blood sugar.
This is a case of false labelling. Note that the two drugs have different modes of action and will have severe effects such as hypoglycemia and death if used interchangeably.
AUGUST 15: MEDICINE QUALITY ALERT: CLASS I MEDICINE RECALL OF S-PRAZO (ESOMEPRAZOLE MAGNESIUM DELAYED-RELEASE CAPSULES 40MG) BATCH NO SPZ-302.
This recall was initiated voluntarily. The drug, S-Prazo, is used in the treatment of ulcers and acidic related conditions. It can also be used to eradicate H Pylori.
Toxicology view: A mix up in packaging mainly will concern us on wrong dosage, leading to either overdose or underdosing. Also, it may cause wrong molecule dispensation and wrong drug expiry time leading to poor outcomes of management.
December 5
QUARANTINE ORDER FOR SUSPECTED SUBSTANDARD FLURASTED 500 INJECTION BATCH NO. HHP24017
A flustrated 500 mg injection is used to treat some types of cancer. This was the second cancer drug that was being recalled in a single year. It was recalled because there were signs of particles in the injectable medicinal product.
Toxicology view: Presence of particles in a drug shows contamination in the manufacturing process. This poses a risk of introducing harmful substances into the bloodstream and this also can affect drug delivery on site of injection.
DECEMBER 11: PUBLIC ALERT: RECALL OF SUBSTANDARD SURE LUBRICATED CONDOMS DOTTED, MANUFACTURED BY INDUS MEDICARE PRIVATE LIMITED.
PPB mentioned that the batch that was recalled did not adhere to the specifications for the freedom from holes test. The United Nations Fund for Population Activities demands that condoms should have physical property requirements like burst volume and pressure, freedom from holes and package integrity. Condoms with visible holes of less than 25 mm from the open end are not considered defective in this test.
Toxicology view: This is a manufacturing defect of having a porous condom. In essence, condoms must be leakage-free to minimise chances of causing a pregnancy and spreading STIs.
How to verify if drugs are real or fake
a. Misspellings of the product name, poor print quality, or
inconsistencies in logos, colours, or font sizes on packaging and
labels.
b. Any noticeable differences in the medicine’s physical appearance, including size, colour, shape, taste, or smell, compared to what they are accustomed to.
c. Any changes in the medicine’s appearance should be confirmed with a pharmacist.
d. Compromised packaging—medications should be sealed in their original manufacturer's packaging. If the packaging is open, appears tampered with, or seems unusual, consult a pharmacist.
e. Some manufacturers incorporate security features such as
holograms and QR codes on the packaging, which patients can
verify.
f. Always purchase medicines from licensed pharmaceutical outlets.
Counterfeit drugs: A global problem
Dr Joseph Wahome Mukundi
For years, the number of counterfeit medications that have made their way into trusted pharmacies and subsequently to patients’ medicine cabinets has been on the rise.
These are drugs or products that have been deliberately and fraudulently produced or mislabeled with respect to identity or source to make it appear to be a genuine product. Counterfeit medications include drugs that contain no active pharmaceutical ingredient (API), an incorrect amount of API, an inferior-quality API, a wrong API, contaminants, or repackaged expired products. Some counterfeit medications may even be incorrectly formulated and produced in substandard conditions.
Imagine a scenario in which a patient takes medication for a life-threatening illness, only to become aware later that the doses contained no APIs. It is estimated that this misfortune has occurred with thousands of people worldwide and continues to happen. The growing issue of counterfeit medications is a concern not only for the patient, but also for pharmacists and pharmaceutical companies.
A WHO study revealed that nearly half (48.7 per cent) of the documented cases of drug counterfeiting were reported in developing countries of the Western Pacific (China, the Philippines, and Vietnam), followed by developing countries grouped within WHO’s Regional Office for Africa, with 18.7 per cent. The industrialised areas of WHO’s Regional Office for Europe came in third, with 13.6 per cent of reported cases.
They explain that most US counterfeit medications are purchased online. However, others have infiltrated legitimate supply chains.
Counterfeiting can apply to both branded pharmaceuticals and their less expensive generic counterparts. In fact, generic drugs are sometimes confused with counterfeit medications, which may pose an obstacle to the widespread use and acceptance of generic medications. Moreover, any impact on generic-drug use is potentially far-reaching.
Effects of using falsified drugs
1. Falsified and substandard drugs may contain toxic doses of dangerous ingredients and cause mass poisoning.
2. Poor quality medicines compromise the treatment of chronic and infectious diseases; causing disease progression, drug resistance and death.
3. As chronic diseases increase in low- and middle-income countries, so will the need for reliable medicines.
4. Substandard and falsified medicines encourage drug resistance, threatening the health of populations today and in the future.
Mr Wahome is a consultant toxicologist and chief officer health Meru County
[email protected]