Premium
AstraZeneca 'may have used old data' in trial, warns US regulator
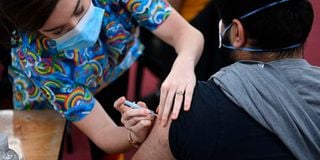
A health worker administers a dose of the AstraZeneca/Oxford vaccine at the Fazl Mosque in southwest London on March 23, 2021. P US health officials have claimed that AstraZeneca may have used "outdated information" to show that its vaccine is highly effective.
British-Swedish drug giant AstraZeneca has once again found itself caught in public doubt over its vaccine, following allegations by US health officials that the company may have used "outdated information" to show that the jab was highly effective.
Hours after AstraZeneca reported that its Covid-19 vaccine provided strong protection among adults, the National Institute of Allergy and Infectious Diseases (Niaid) said it was worried the trial results have given only a partial picture.
In an unusual statement released early Tuesday, experts from Niaid raised concerns that the results announced by AstraZeneca indicating that its vaccine was 79 per cent effective in preventing against Covid-19 for people with symptoms may have been based on “an incomplete view of the efficacy data” from a clinical trial and relied on “outdated information,” throwing another curveball in the saga surrounding the company’s vaccine.
Jab safe
The company also said its vaccine, AZD1222, was found to be 100 per cent effective in preventing severe illness and hospitalisation. In addition, the results drawn from a study of more than 32,000 participants in Chile, Peru, and the US showed the jab was safe.
But the Niaid, one of the 27 institutes and centres that make up the National Institutes of Health (NIH), said it was worried the trial results have given only a partial picture, after being informed about the data questions by the data and safety monitoring board (DSMB) auditing the trial. DSMBs consist of independent medical experts who provide an extra screen of data produced from clinical trials.
Review data efficacy
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” Niaid said.
In an interview on Tuesday morning with American health-oriented news website STAT, Anthony Fauci, the head of Niaid, said the DSMB raised concerns because it felt the results in the AstraZeneca press release looked more favourable than more recent data from the vaccine study had shown.
Stunned
“I was sort of stunned,” Fauci said. “The data safety and monitoring board were concerned that the data that went into the press release by AZ was not the most accurate and up to date data. That is what the DSMB communicated to AZ in a rather harsh note. Having seen that letter, we could not just let it go unanswered.”
Responding to the concerns, AstraZeneca said in a statement that it would “immediately engage” with the DSMB to share primary analysis with the most up to date efficacy data within 48 hours.
"The numbers published yesterday were based on a pre-specified interim analysis with a data cut-off of 17 February," the company said.
"We have reviewed the preliminary assessment of the primary analysis and the results were consistent with the interim analysis. We are now completing the validation of the statistical analysis.”
Side effects
The vaccine has faced some challenges that have eroded confidence in its shots following news of possible rare, serious side effects.
Last week, at least a dozen European countries paused the administration of the vaccine following reports of rare strokes known as cerebral venous sinus thromboses (CVST) and clotting disorders in people who had received it.
But on Thursday, the European Medicines Agency (EMA) said that while it could not rule out that the symptoms were connected to the vaccine, the benefits strongly outweighed the risks, and, as a result, the countries resumed using the Covid-19 vaccine.
Three African countries — the Democratic Republic of Congo, Mali, and Cameroon — put the rollout of the AstraZeneca vaccine on hold as a precaution following the EU safety concerns.
The company also faced challenges in South Africa which early this year paused its rollout of the vaccine after a small study suggested it offers minimal protection against mild and moderate infection from the variant known as B.1.351.