News
Premium
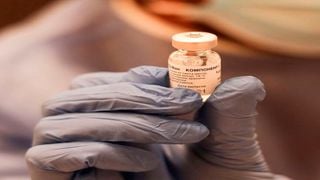
Kenya approves use of controversial Russian Covid-19 vaccine
Russia has announced that Kenya has approved its Sputnik V Covid-19 vaccine.
Russian Direct Investment Fund (RDIF), the country's sovereign wealth fund, on Wednesday announced the approval of the use of Sputnik V vaccine in Kenya.
This comes weeks after the African Union's vaccine task force revealed that Russia had offered AU 300 million doses of the Covid-19 vaccine, spicing up the deal with a financing package for countries wanting to secure the shots.
The AU announced that the Russian vaccine will be available for 12 months starting in May.
RDIF, which has been tasked to market the vaccine abroad, said some deliveries could start in May but most would be from June.
Efficacy of the vaccine
The efficacy of the vaccine is rated at 91.6 per cent as per data published in the Lancet, one of the world's most trusted medical journals.
This means that Sputnik V is one of three vaccines in the world with efficacy of over 90 per cent.
"With the authorisation of Sputnik V in Kenya there are now 10 African nations that have registered the Russian vaccine. Sputnik V is approved in 47 countries around the world with more registrations expected in coming weeks. It is truly a vaccine for all humankind and one of the best solutions to fight the pandemic," Kirill Dmitriev, the chief executive officer of RDIF said on the vaccine's official website.
Contacted by the Nation, Dr Willis Akhwale, Kenya's vaccine advisory taskforce chair, however, said that he was not privy to any official decision from the Ministry of Health (MoH) on the vaccine.
"Their request was with the Pharmacy and Poisons Board (PPB) and maybe they have been engaged. It is possible but I don't have the information, you know at that level it is between the manufacturer and PPB," Dr Akhwale said.
A highly placed official at MoH who sought anonymity said he had not received any information as well and asked for time to consult PPB.
According to the Kenyan drugs regulator, the Russian vaccine application for Emergency Use Authorisation has been evaluated and approved. But PPB said this does not imply registration.
"The initial application was submitted on 05th February 2021. First round assessment on quality, efficacy and safety was initiated on 08th February 2021 (with rolling submission of data) and completed on 24th February 2021 and communication sent out the same day.
The application was not successful and second round of assessment following the applicant's response to outstanding questions was completed on 5th March during which it met all requirements," PPB said in an email response to Nation.
PPB added that in reviewing Sputnik V it considered all aspects of quality, safety and efficacy and found that it is wholesomely safe but MoH has not yet decided to use Sputnik V vaccine as it is not included in the national vaccination program.
According to RDIF, Sputnik V is registered for emergency use authorisation by World Health Organization (WHO).
It is the second most globally accepted vaccines in terms of approvals issued by government regulators.
Its storage temperature is +2+8 C which means it can be stored in a conventional refrigerator without need for cold-chain infrastructure and poses no strong allergies.
"I am not sure we will provide some extra information to speak frankly," Alexei Urazov, a director at RDIF told Nation in response to questions on Russia's vaccine deal with Kenya.
The vaccine is being used in Russia, Belarus, Argentina, Bolivia, Serbia, Algeria, Palestine, Venezuela, Paraguay, Turkmenistan, Hungary, UAE, Iran, Republic of Guinea, Tunisia, Armenia, Mexico, Nicaragua and Republika Srpska (entity of Bosnia and Herzegovina).
Other countries are Lebanon, Myanmar, Pakistan, Mongolia, Bahrain, Montenegro, Saint Vincent and the Grenadines, Kazakhstan, Uzbekistan, Gabon, San-Marino, Ghana, Syria, Kyrgyzstan, Guyana, Egypt, Honduras, Guatemala, Moldova, Slovakia, Angola, Republic of the Congo, Djibouti, Sri Lanka, Laos, Iraq and North Macedonia.
Developers of the vaccine say they are working with AstraZeneca on a joint clinical trial to improve the efficacy of the AstraZeneca vaccine.
Guinea is in talks and aims to acquire 400,000 doses, according to reports from the country's a health ministry.
Despite Russia's efforts to export the vaccine, media reports indicate authorities are struggling to roll out a nationwide vaccination strategy.
Three days ago the European Medicines Agency (EMA) cautioned European Union (EU) members to delay granting national authorisation for the Russian-developed vaccine until the agency finishes its safety review.
"We need documents that we can review. We also don't at the moment have data about vaccinated people," EMA managing board chief Christa Wirthumer-Hoche told Austrian broadcaster ORF.
"It is unknown. That's why I would urgently advise against giving a national emergency authorisation," she explained.
Health Ministry acting Director General Dr Patrick Amoth was not available for comment.





