Pharmacy board says it approved local use of Sputnik V vaccine
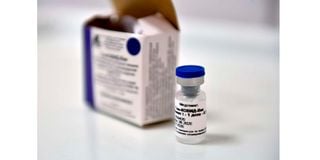
A vial with Russia's new coronavirus vaccine is seen prior to a vaccination of a volunteer in a post-registration trials, Moscow, September 10, 2020.
What you need to know:
- Dinlas Pharma EPZ Ltd was authorised to import 50,000 vials for Component I and another 50,000 vials for Component II of the vaccine.
The Pharmacy and Poisons Board has told Parliament it approved the use of Russia’s Covid-19 vaccine, Sputnik V.
Dr Ahmed Mohamed, the director in-charge of health products and technologies at the board, in response to a query from Rarieda MP Otiende Amolo, said the application for importation was made on March 12 by Dinlas Pharma EPZ Ltd.
The company was authorised to import 50,000 vials for Component I and another 50,000 vials for Component II of the vaccine.
“The board approved the vaccine for use by the general public,” Dr Mohamed said. “However, the importer imported 50,000 vials for the first component and 25,000 vials for the second component.”
Although the government has since banned the importation, distribution and administration of the vaccine by the private sector, Dr Amolo wrote to the PPB, the regulator, seeking information on the presence of the Russian-made vaccine in the country.
Dr Amolo invoked Article 35 of the Constitution and the Access to Information Act, demanding answers from the regulator as to whether the vaccine had been approved by the World Health Organisation or any other international organisation, as is the standard practice, before use.
“The board carries out its functions through resolutions made in its meetings and which meetings the law requires you to keep a record of,” Dr Amolo said in the letter addressed to the board chair Dr Jackson Kioko.
Dr Amolo wanted to know the purpose for which the Sputnik vaccine was approved in Kenya, when the approval was done, and the minutes of the meeting that approved the importation.
He further sought data on the exact quantity of the vaccine that was imported and whether any tests were conducted as to its efficacy, when and where.
The PPB, in its response, said that tests for the efficacy of the vaccine were conducted before the authorisation.
“The efficacy studies was conducted by the manufacturer and the data provided to the Board by the applicant at the time of application for emergency use authorization,” said Dr Mohamed.





