Premium
Russian Sputnik V vaccine is in Kenya legally, says board
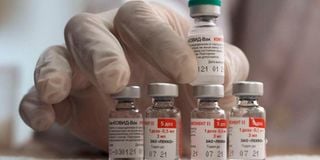
Vials of the Russian Sputnik V Covid-19 vaccine are pictured during vaccination in Gaza City on March 25, 2021.
The Pharmacy and Poisons Board (PPB) has confirmed to the Nation that the Sputnik V vaccine is legally in the country and that only one hospital has so far been accredited to administer the vaccine.
“Only one from all that had applied to administer the vaccine passed the test, Bliss Westlands, has been given clearance to administer the vaccine to any Kenyan. We are going to accredit more but only those who pass the test,” says the board.
It states that for Sputnik V 1 which is given as a first dose, the hospitals must prove that they can store the vaccine at -18 degrees celsius while for the second dose Sputnik V 2, can be stored between 2 to 8 degrees celsius.
“It is not that the vaccine is illegal, we approved the vaccine and it is safe, our people are on the ground doing surveillance for hospitals and those that meet the threshold will be accredited,” says the Board.
From the inside sources, the distributor of the vaccine has everything that the government had requested from them including training of the health care workers and taking private indemnity in case of any eventuality.
On Monday this week at exactly 4:34 pm, a tax payment of 8.5 million was made to Kenya Revenue Authority a day after 75,000 doses of Russian vaccine were delivered at Mombasa ports.
Import Declaration Fee
The KRA domestic tax receipt with the customer reference number of a121-00448 cleared the company for six taxes including Kenya Railways Development Levy which they paid Sh3.1 million, Import Declaration Fee of Sh5.4 million and concession fees.
Import Declaration Fee, is a levy charged by the Kenya Revenue Authority on all imported goods. In Kenya, the IDF fee is at a rate of 2.25 per cent of the Insurance and Freight (CIF) value
Other taxes which were not charged but were cleared included VAT import, import duty and excise duty.
This was a payment made by Dinlas Pharma EPZ Limited for the Sputnik V, the Russian coronavirus vaccine that was delivered on Sunday last week.
After clearing with the KRA, according to the documents seen by the Nation, the latter was then instructed to get an import release note from the ports.
This they did, the note was processed and signed by an officer identified as Celestine on March 22.
In the clearance note, the ICCD number 00300955 indicated that the consignment was in good order and condition.
The two certificates indicated that the goods were in the country legally.
All this happened after the PPB approved the emergency use of the vaccine in the country, authorising Dinlast to import the vaccine.
The Board cleared the company to import the vaccine, the clearance by the PPB cannot happen without the knowledge of the mother ministry, Ministry of Health.
On March 24, the PPB via its official twitter handle, said it had given Emergency Use Authorisation to Sputnik V vaccine after a successful evaluation process and after it met all the requirements.
The PPB had also announced that any company planning to import Covid-19 vaccine into the country had to sign an indemnity agreement before being cleared
Failure to sign this agreement, the manufacturer will not be given permission to import.
The move was to ensure that the manufacturers or any local agents importing the vaccine takes responsibility for any adverse effect on patients that might have been vaccinated.
With the restriction and the directive, Dinlas Pharma EPZ Limited on March 12, even before importing the vaccine, applied for Sh201 million professional indemnity through the AAR Insurance Company Ltd for a year starting March 12, 2021 to March 11, 2022.
The indemnity was to cover for adverse reactions due to covid-19 vaccination and with the certificate, the benefits are available in the company.
“It is hereby agreed that the benefits of the policy issued are available at Dinlas Pharma EPZ Limited subject to the terms, conditions and exceptions of the professional indemnity policy issued,” as indicated in the certificate signed by one Mr Robert Ngetich.
From the certificate, it states that the maximum limit per event is Sh1 million and one year aggregate Sh200 million.
The board added that in reviewing Sputnik V it considered all aspects of quality, safety and efficacy and found that it is wholesomely safe but MoH has not yet decided to use the vaccine as it is not included in the national vaccination programme.
All these were done with the knowledge of PPB officials who were aware of the arrival of the vaccines.
However, on Thursday last week, two Ministry of Health officials denied knowing that the vaccine was authorised for use in the country and that they were not aware that the vaccine had arrived hence raising safety concerns over the planned sale of the jab to the public.
The head of immunisation at the Ministry, Dr Collins Tabu said he was not aware of the Sputnik V vaccine’s existence in the country, even as the Pharmacy and Poisons Board (PPB) said it had only approved its importation but not distribution.
Dr Willis Akhwale, the vaccine advisory task force chairman, said he is not privy to any discussions with anyone at the Health ministry on the arrival of the vaccine in the country.
Private health facilities are already registering Kenyans for the vaccine after the vaccine's arrival was made public by Russian Direct Investment Fund (RDIF) on their official twitter handle.
Mr Nishant Mishra, a senior official at Dinlas Pharmaceuticals, the distributor, yesterday said that the vaccine has been imported with the full knowledge and clearance of the PPB.
“We used Freight in Time Ltd as our clearing agent and we had gotten the clearance. We are using the Freight in Time’s cold room to (store it) at the required temperature levels. We have a tracker to monitor the temperature round the clock,” Mr Mishra told Nation.
When the Nation asked the PPB why the vaccines have not been distributed to private hospitals in the country, one official who sought anonymity said that the distribution clearance was part of the Ministry of Health.
“Ours is to deal with the safety and ensuring that they meet all the requirements after which will give distribution clearance since they deal with distribution of health products and pricing, not the board," board said.
Before the distribution of the vaccine clearance, the company was to ensure users’ safety, which private distributors must meet before approval.
The distributor also needs to register users on the government-run Chanjo site, where everyone getting vaccinated has their details filed, to enable the ministry to track the number of people taking the vaccine and any side effects.
The distributor also has to sign a technical agreement stipulating the responsibilities of all the parties involved in its administration and training health care workers on administration of the vaccine.
From the conversation that the Nation is privy to, Dinlas Pharma EPZ Limited on Friday through the Ministry of Health trained health care workers from 27 private health facilities.
The vaccine will be administered by private health facilities in the country at Sh8, 000 per dose.
“We have met all the requirements and conditions and whoever can afford to pay for the vaccine should be allowed to,” says Mr Mishra
“The vaccine will be distributed through the health facilities’ networks. Freight in Time Ltd will help us do the distribution because they have the facilities to transport the vaccine at required temperatures. “We will also be ensuring that the health facilities that receive the vaccine have the cold storage facilities,” said Mr Mishra.
Authorisation Letter
In a conversation between Russian Direct Investment Fund written to the Cabinet Secretary Mutahi Kagwe on March 24 stated that given that the delivery came from an authorised partner Human Vaccine will have an import license or the delivered batches of the vaccine.
The letter titled Authorisation Letter for sale and distribution Sputnik V Adenovirus Vector Vaccine for Covid-19 said that the company had been informed that three batches of the vaccine had been delivered to Kenya.
“As we know, in addition to the already provided authorisation of the vaccine, each batch of imported vaccines shall be additionally approved by PPB,” says the letter.
“We have done everything that the government asked us to do including training but if the interest to distribute the vaccine is not there, then we can take it somewhere else where it is needed. This is not about safety and we are ready to do anything that is required of us,” said an official at the company who sought anonymity.
The Sputnik V coronavirus vaccine gives around 92 per cent protection against Covid-19, late-stage trial results published in The Lancet revealed. The analysis also showed that the shot is safe and offers complete protection against hospitalisation and death.
Different vaccines have different attributes including storage requirements, their efficacy, dosing regimens, and manufacturing platforms.
For Sputnik V, its storage temperature is -18 degrees Celsius. The jab is also said to pose little risk of allergies. The two doses of the Russian vaccine are given 21 days apart unlike the eight weeks apart for the Oxford AstraZeneca vaccine.
The AstraZeneca vaccine is already in use in the country after the delivery of the 1.2 million doses under the Covax facility. Over 90,000 people have been vaccinated countrywide so far.
The Africa Centers for Disease Control and Prevention (AfricaCDC), African Union vaccine acquisition task force, last month secured 300 million doses of Russia’s Sputnik V vaccine on behalf of the continent.
In a statement, (AfricaCDC) said it is “tremendously proud” to offer the doses from May to the 54 member States.
“We are grateful to receive the Sputnik V vaccines from the Russian Federation and tremendously proud to be able to offer them on the AMSP for our AU Member States,” Africa CDC director John Nkengasong said adding that about 26 countries have already submitted their applications to acquire 180 million doses of these vaccines.