Premium
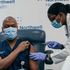
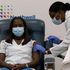
Scientists give nod to Pfizer-BioNTech vaccine
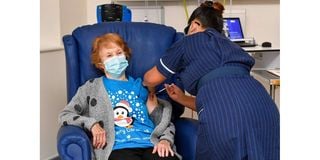
A nurse administers the Pfizer-BioNtech Covid-19 vaccine to Margaret Keenan , 90, at University Hospital in Coventry, central England on December 8, 2020.
Scientists have endorsed the Pfizer and BioNTech vaccine — BNT162b2, days after it was approved for emergency use in the United Kingdom.
On Friday, the vaccine was also approved for emergency use in the United States.
In a peer reviewed study released late last week and published at the The New England Journal of Medicine, the scientists from various institutions found that a two-dose regimen of BNT162b2 (30 μg per dose or 0.3 ml volume per dose), given 21 days apart was safe and 95 per cent effective against Covid-19. “All the trial data were available to all the authors, who vouch for its accuracy and completeness and for adherence of the trial to the protocol,” states the research report. The vaccine, which was tested in persons aged 16 years or older, was found to be safe after up to two months observation, similar to that of other viral vaccines.
Adults aged 16 years or older, who were healthy or had stable chronic medical conditions, including but not limited to HIV, hepatitis B virus or hepatitis C virus infection, were eligible for participation in the trial.
But, those with a medical history of Covid, treatment with immunosuppressive drugs or diagnosis with low immunity were not allowed to participate.
The researchers analysed vaccine efficacy among participants with hypertension separately, but the results were consistent with the other subgroup analyses.
But safety results for participants infected with HIV (196) will be analysed separately as per protocol, according to the study report. But the research report fails to “address the prevention of Covid-19 in other populations such as younger adolescents, children and pregnant women.