We didn’t approve Russian vaccine, ministry now says
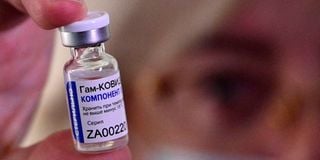
A medical worker displays a vial with Sputnik V vaccine during the vaccination of medics at a clinic in the far eastern city of Vladivostok on December 15, 2020.
What you need to know:
- Private health facilities have already started registering Kenyans for the jab.
- Distributor insists the Pharmacy and Poisons Board (PPB) okayed importation and distribution of the Sputnik V vaccine.
Two Ministry of Health agencies have denied authorising the reported release of Russian-made Covid-19 vaccine, Sputnik V, into some private Kenyan hospitals, raising safety concerns over the planned sale of the jab to the public.
The head of immunisation at the Ministry, Dr Collins Tabu, on Wednesday said he was not aware of the Sputnik V vaccine’s existence in the country, even as the Pharmacy and Poisons Board (PPB) said it had only approved its importation but not distribution.
Private health facilities such as the Nanyuki Cottage Hospital had by on Wednesday already started registering Kenyans for uptake of the jab at a reported cost of Sh8,000. The World Health Organization (WHO) has not yet authorised emergency use of Sputnik V, even though it’s already in use in multiple countries across the globe.
Mr Nishant Mishra, a senior official at Dinlas Pharmaceuticals, the distributor, yesterday said that the vaccine has been imported with the full knowledge and clearance of the PPB.
“We used Freight in Time Ltd as our clearing agent. We are using the Freight in Time’s cold room to (store it) at the required temperature levels. We have a tracker to monitor the temperature round the clock,” Mr Mishra told Nation.
Distribution guidelines
A PPB official who did not wish to be named admitted that the agency had authorised the importation of Sputnik V, but claimed the board was not aware of its distribution across the country.
“It’s not in the market because we’re still developing the guidelines, which they have to meet before it is finally authorised for usage,” the official said.
There are conditions, the official said, which are supposed to ensure users’ safety, which private distributors must meet before approval.
The distributor also needs to register users on the government-run Chanjo site, where everyone getting vaccinated has their details filed, to enable the ministry track the number of people taking the vaccine and any side effects.
The distributor also has to sign a technical agreement stipulating the responsibilities of all the parties involved in its administration.
Deal with WHO
The government signed an agreement with WHO waiving the right to sue any pharmaceutical company for any adverse effects of the AstraZeneca vaccine, which is currently the only Ministry of Heath-registered Covid-19 immunisation in the country. The Senate Health Committee has also denied knowledge of the existence of the Russia Sputnik V vaccine in the country.
While inspecting the National Vaccine Depot in Kitengela yesterday, Committee chairperson Sabina Chege, said parliament is only aware of the AstraZeneca vaccine.
Different vaccines have different attributes including storage requirements, their efficacy, dosing regimens, and manufacturing platforms.
Little risk of allergies
For Sputnik V, its storage temperature is 2 to 8 degrees Celsius, which means it can be stored in a conventional refrigerator without need for cold-chain infrastructure.
The jab is also said to pose little risk of allergies. The two doses of the Russian vaccine are given 21 days apart unlike the eight weeks apart for the Oxford AstraZeneca vaccine.
Nanyuki Cottage Hospital CEO Anup Das told Nation yesterday that the vaccine would be available from today at a cost of Sh8,000 per dose.
“We got the vaccine through the distributors who imported with the right authorisation and the vaccine is being stored by a logistics company in Nairobi under the required temperature,” said Dr Das.
Dinlas Pharmaceutical denies that it has made plans to release the jab, even as it insist that all its approvals were procedural.
“We obtained an Emergency Use Authorisation from the Pharmacy and Poisons Board on March 9 and we also have all other documents, licenses and batch releases.
“The vaccine will be distributed through the health facilities’ networks. Freight in Time Ltd will help us do the distribution because they have the facilities to transport the vaccine at required temperatures.
“We will also be ensuring that the health facilities that receive the vaccine have the cold storage facilities,” said Mr Mishra.
Slow uptake
The AstraZeneca vaccine is already in use in the country after the delivery of the 1.2 million doses under the Covax facility. About 56,000 people have been vaccinated countrywide so far. Speaking to Nation yesterday, Dr Willis Akhwale, the vaccine advisory task force chairman, said he is not privy to any discussions with anyone that arrived at such a decision at the Health ministry.
“The CEO has been directed to publish requirements of the distributors who want to distribute the vaccines,” he said.
Sputnik V is in use in Russia, Belarus, Argentina, Bolivia, Serbia, Algeria, Palestine, Venezuela, Paraguay, Turkmenistan, Hungary, UAE, Iran, Republic of Guinea, Tunisia, Armenia, Mexico, Nicaragua and Republika Srpska (entity of Bosnia and Herzegovina).
Other countries where the vaccine is in use are Lebanon, Myanmar, Pakistan, Mongolia, Bahrain, Montenegro, Saint Vincent and the Grenadines, Kazakhstan, Uzbekistan, Gabon, San-Marino, Ghana, Syria, Kyrgyzstan and Guyana.
It’s also in Egypt, Honduras, Guatemala, Moldova, Slovakia, Angola, Republic of the Congo, Djibouti, Sri Lanka, Laos, Iraq and North Macedonia.
Two weeks ago, the European Medicines Agency cautioned European Union members to delay granting national authorisation for the Russian-developed vaccine until the agency finalises its safety reviews.
“We need documents that we can review. We also don’t at the moment have data about vaccinated people,” EMA Managing Board chief Christa Wirthumer-Hoche told Austrian broadcaster ORF.
What you need to know about vaccines
With Covid-19 vaccination underway in Kenya, experts from Doctors for Healthy Living will answer all your questions about Covid-19 vaccines. Today, Dr Moses Mwangi, a vaccine expert, responds to your questions.
What is a vaccine?
A vaccine is a weakened form or a part of a disease-causing agent with the ability to trigger an immune response, but with no ability to cause disease. The response leads to production of antibodies whose job is to fight the invading germ.
How do vaccines work?
When someone gets vaccinated against Covid-19, the body reacts as if it had been infected with the real virus. The vaccine leaves a memory in the body giving it the ability to quickly make the antibodies in case the individual contracts coronavirus.
The Covid-19 vaccines were developed so fast, are they really safe?
Unknown to many, there are several viruses that are closely related to the novel coronavirus that causes Covid-19. The first one, which was identified in 2003 was named, Severe Acute Respiratory Syndrome (SARS) and almost caused a pandemic but was quickly controlled though it caused several hundred deaths and made thousands of others sick. In 2012, another coronavirus named Middle East Respiratory Syndrome (MERS) was identified but did not wreak much havoc. The search for a vaccine against coronaviruses therefore begun in earnest and has been going on for well over 15 years. All the Covid-19 vaccines that have been approved around the world, including Kenya, are very safe.
They have been tested on thousands of people in well designed and controlled clinical trials. The Oxford-AstraZeneca vaccine in use in Kenya has been used on millions of people in the UK with no serious side effects reported.
Compiled by Chrispine Onyango. To send your questions or get more information, e-mail [email protected] or call: 0721494599