WHO approves new tuberculosis drug for children, adolescents
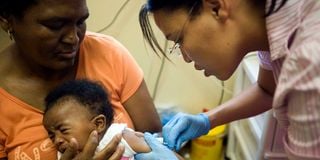
A nurse injects a child with aTB vaccine. WHO’s consolidated guidelines recommend a four-month regimen for children and adolescents below 16 years with non-severe drug-susceptible TB.
What you need to know:
- The new regimen, recommends that children and adolescents between three months and 16 years of age with non-severe TB (without suspicion or evidence of multidrug- or rifampicin-resistant TB (MDR/RR-TB) be given the four months treatment regimen.
- In children with MDR/RR-TB aged below six years, an all-oral treatment regimen containing bedaquiline is recommended while in children below three years with MDR/RR-TB, delamanid may be used as part of longer regimens.
Children and adolescents can now access new and simple ways of treating multidrug-resistant tuberculosis, thanks to the World Health Organisation (WHO).
WHO’s consolidated guidelines recommend a four-month regimen for children and adolescents below 16 years with non-severe drug-susceptible TB.
This will be used in treating multidrug-resistant tuberculosis (MDR-TB)/rifampicin-resistant TB (RR-TB), a type of tuberculosis whose bacteria can’t be treated using the two main drugs — isoniazid and rifampin. The international agency’s operational handbook provides dosing guidance for bedaquiline and delamanid for children weighing down to three kilogrammes and instructions on the preparation of the formulations of both medicines. The use of bedaquiline as part of shorter or longer regimens for children of all ages to treat MDR/RR-TB and the use of delamanid as part of longer regimens for children of all ages to treat MDR/RR-TB.
Bedaquiline is a component of the nine-month all-oral regimen, which is the treatment of choice for eligible children and young adolescents aged under 14 years with MDR/RR-TB, rather than longer (18-month) regimens.
Delamanid, on the other hand, is always used as part of longer individualised regimens for people with MDR/RR-TB, including children and adolescents, who are not eligible for the nine-month all-oral regimen.
“The information notes provide clear and simple instructions for clinicians and caregivers on the use of these dispersible child-friendly formulations, but also on the use of adult tablets when child-friendly formulations are not available,” said Dr Tereza Kasaeva, director of WHO’s Global Tuberculosis Programme
“Child-friendly formulations of bedaquiline and delamanid are now available. We need to ensure that children and adolescents of all ages can benefit from these new drugs and innovative regimens," she adds.
New evidence from a recently completed trial on the shortened treatment of drug-susceptible TB in children and adolescents has paved the way for new recommendations on shorter regimens for this group.
The SHINE trial (Shorter Treatment for Minimal Tuberculosis in Children) was the first and only large phase-three trial to evaluate the duration of TB treatment in children with non-severe drug-susceptible TB.
Children and young adolescents aged below 16 years were treated with rifampicin, isoniazid, pyrazinamide with or without ethambutol using WHO-recommended doses, appropriate for paediatric dosing.
The 2022 guidelines done to improve TB case detection and treatment outcomes in children and adolescents with TB using effective models of care is making it possible to build all-oral regimens for everyone with MDR/RR-TB, irrespective of age. The recommendation will also contribute to reduction in TB-related sickness and death in children and adolescents in line with global targets.
Data from the World Health Organization shows that Kenya is one of the 22 countries that contribute to about 80 per cent of world TB cases. It ranks 15th out of the 22 most vulnerable countries globally.
Multidrug-resistant tuberculosis is mostly blamed on human-made problems and emerging due to poor management of TB.
From the WHO data, around 25 000–32 000 children develop MDR/RR-TB every year.
In 2020, about 3, 234 children (aged below 15 years) were started on second-line treatment for MDR/RR-TB representing only 2.5 per cent of the total number of persons with MDR/RR-TB initiated on treatment.
“With better options for treatment now available, efforts to find children with drug-resistant forms of TB need to be scaled up so that even the youngest children can benefit from better and shorter treatment and lead healthy and productive lives,” Dr Kasaeva said.
The new regimen, recommends that children and adolescents between three months and 16 years of age with non-severe TB (without suspicion or evidence of multidrug- or rifampicin-resistant TB (MDR/RR-TB) be given the four months treatment regimen. In children with MDR/RR-TB aged below six years, an all-oral treatment regimen containing bedaquiline is recommended while in children below three years with MDR/RR-TB, delamanid may be used as part of longer regimens.
Bedaquiline may also be included in longer MDR-TB regimens for patients aged 6–17 years.
Recommendation on the use of delamanid and bedaquiline in children aged less than three years has not been possible in the past due to a lack of evidence, particularly on safety and tolerability. It made it challenging for clinicians to design all-oral regimens for children under the age of three years, especially those with limited drug choices
The use of delamanid in children below three years was therefore identified as a gap to be addressed.
“Appropriate treatment of children with MDR/RR-TB with a WHO recommended regimen is an important step in ensuring a successful treatment outcome as well as preventing the acquisition of further drug resistance,” says WHO.
The global health agency advised high TB burden countries to decentralise TB services in children and adolescents with signs and symptoms of the disease and/or in those exposed to it.





