Premium
Explainer: How Kenya and Africa can beat MPox
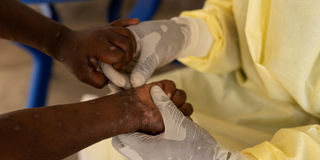
A nurse takes a sample from a child declared a suspected case of Mpox - an infectious disease caused by the monkeypox virus that sparks off a painful rash, enlarged lymph nodes and fever.
Barely over a year after the World Health Organization (WHO) declared that mpox was no longer a public health issue of international concern, it is back in the news. This time with a diversity of variants, new modes of transmission and new populations infected.
In this interview, Oyewale Tomori, a professor of virology, explains why the declaration of mpox as a global public health emergency is connected with the failure of African governments to properly fund disease surveillance activities and create an environment for their deeply experienced health workers to function. He also sheds light on what it takes to stop a disease outbreak.
What’s the advice of WHO on managing mpox?
The WHO strategic framework for enhancing prevention and control of mpox 2024-2027 highlights the need for countries to take immediate action to ensure that surveillance, testing, treatment and vaccination responses are established and integrated with other health programmes.
This includes disease surveillance, sexual health services, risk communication and community engagement, primary healthcare, immunisation and other clinical services.
The control and containment of the 2022-2023 multi-country outbreak within a year in non-endemic countries outside Africa was achieved through a combination of:
surveillance (detection and laboratory confirmation of cases, contact tracing)
isolation of cases
infection protection and control
targeted vaccination of high-risk individuals.
African health workers have battled various disease outbreaks for more than half a century. What lessons have been learnt?
African health workers have over 50 years of experience dealing with different and various outbreaks of yellow fever, Ebola, mpox and COVID-19. They have acquired the relevant expertise and skills to prevent the spread of epidemics.
There are at least four instances in African countries where outbreaks were contained before they escalated to emergencies of concern. These successes were achieved through enhanced surveillance, rapid laboratory confirmation of cases, contact tracing, isolation of cases, as well as awareness campaigns on avoiding contacts with cases. Infection protection and control measures were taken.
In mid-2014, west Africa was in the grip of the largest epidemic of Ebola the world had ever seen. On 20 July 2014, a man infected with Ebola landed in Lagos, Nigeria, a city of 21 million. Infections began to spread immediately.
By the end of the month, the first patient had died, an infected individual had flown to another city and one thousand contacts had been exposed to the virus. And yet, in Nigeria, the outbreak was over in less than three months.
Nigeria stopped Ebola from spreading nationally, and potentially regionally, with effective communication, coordinated response activities and dedicated leadership.
In 2018, Ebola crossed into Uganda through its busy border with the Democratic Republic of Congo. Uganda rapidly mobilised its response teams and activated its health emergency response system, which stopped Ebola from spreading into the country.
Again in 2018, in a rural area of Kenya, a deadly anthrax outbreak was identified and brought under control thanks to a community-based surveillance system and a trained volunteer who acted quickly.
Health officials in Akwa Ibom, Nigeria quickly contained, within a month, an outbreak of mpox in 2021. They did this through strong collaboration with national rapid response teams, identifying and correcting weaknesses in the response and providing education and recommendations to improve future responses.
In all these instances, no vaccine was used to contain these outbreaks. Successful containment resulted from the rapid institution of disease surveillance to detect, diagnose, isolate and treat cases, and contact tracing among engaged, involved and aware communities.
Why aren’t these lessons being applied?
There are two main reasons that African governments have not taken advantage of their skilful human resources with relevant disease control experience.
Firstly, they have not provided adequate and sustained funding for an efficient disease surveillance system. Such a system needs to be backed by reliable diagnostic laboratory service for timely detection and confirmation of yellow fever, Ebola, Lassa fever, cholera and other diseases. These diseases often start as sporadic cases, later spreading and becoming large outbreaks.
Secondly, they have not created an enabling environment to carry out disease surveillance activities, such as contact tracing and isolation of cases. Disease surveillance is needed to contain the sporadic cases.
For instance, in the 2014-2016 Ebola outbreak, which primarily affected Guinea, Liberia and Sierra Leone, it took months to identify the disease after a first cluster of cases in December 2013. It took almost three years to contain, and claimed thousands of lives.
The epidemic exposed the dire consequences of weak health systems, poor disease surveillance, an initially lethargic response, and inadequate community engagement.
It would be far cheaper and more cost effective to provide funds for such a system than to respond to an epidemic with fatal consequences. A neglected disease outbreak can become the catalyst for gross domestic poverty.
What is the role of vaccines in preventing diseases?
Vaccines work by stimulating the immune system to recognise and mount a response against specific pathogens, such as viruses or bacteria.
They are designed to mimic the infection without causing the disease itself, allowing the body to develop immunity against the targeted pathogen. Vaccines play a critical role in controlling and preventing epidemic diseases by boosting immunity and reducing the spread of infectious agents.
Great as they are, vaccines cannot take the place of rapid institution of disease surveillance to detect, diagnose, isolate and treat cases. Prevention has always been better than cure.
Are different responses needed for the different variants of mpox?
Not really. However, the mode of transmission – animal-human or human-human (sexual or non-sexual) – will determine the process to prevent or halt transmission and spread of the disease.
These are two clades with variants.
Clade Ia: This is endemic in the Democratic Republic of Congo. It primarily affects children, with a case fatality rate of 3.6% in 2024. Other African countries reporting Clade Ia outbreaks in 2024 include the Central African Republic and the Republic of Congo. Clade Ia has historically been characterised by more severe disease than that associated with Clade II.
Clade Ib: This emerged after September 2023. It is spread through human-to-human transmission. It has spread rapidly in the eastern part of the DRC. The outbreak has primarily affected adults. It is sustained, but not exclusively, through transmission linked to sexual contact and amplified in networks associated with commercial sex and sex workers.
Since July 2024, cases of Clade Ib, linked (in the way it emerged and spread) to the outbreak in the eastern provinces of DRC, have been detected in four countries neighbouring the DRC which had not reported cases of mpox before: Burundi, Kenya, Rwanda and Uganda.
Clade IIa: Reported cases in Cameroon, Côte d’Ivoire, Liberia, Nigeria and South Africa are linked to Clade IIa.
Clade IIb: This virus caused the multi-country outbreak from July 2022 to May 2023. In 2022, mpox entered a new phase when the first case of the disease not associated with travel from Africa was reported in the UK.
This triggered the multi-country outbreak which the WHO declared a global public health emergency of international concern in July 2022. By the time it was declared over in May 2023, 118 countries (7 mpox endemic and 111 mpox non-endemic) had reported a total of 87,377 cases (1,587 in endemic and 87,377 in non-endemic countries).
Oyewale Tomori is a Fellow of the Nigerian Academy of Science.