Premium
India bans export of Covid-19 treatment drug remdesivir
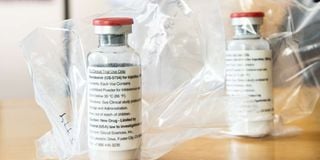
This file photograph taken on April 8, 2020, shows vials of the drug 'remdesivir' during a press conference on Covid-19 medication at The University Hospital Eppendorf (UKE) in Hamburg, northern Germany.
A month after suspending Covid-19 vaccine exports, India has banned the export of an anti-viral drug Remdesivir and its active pharmaceutical ingredients after a record spike in coronavirus cases led to a surge in demand for the drug.
The Indian government Sunday said the ban will be in place in an attempt to balance rising domestic demand.
"Export of injection Remdesivir and Remdesivir Active Pharmaceutical Ingredients (API) is prohibited till the Covid-19 situation in the country improves," the government said in a statement.
India is currently witnessing a recent surge in coronavirus cases. As of April 11, there are 110,008 new active Covid cases. As a result, a sudden spike in demand for Remdesivir, the drug used in the treatment of Covid patients, has emerged with people forming long queues stretching over 2 kilometres to get the drug.
India suspended vaccine exports in March in an attempt to balance surging domestic demand with international orders. The ban on exports means that poorer nations will probably have to wait a few months before receiving their first shots.
Remdesivir is considered a key anti-viral drug in the fight against Covid-19, especially in adult patients with severe complications.
Shorten recovery period
In November last year, the Kenyan government gave a permit to hospitals for the use of the antiviral medication found to shorten the recovery period of Covid-19 patients.
Through the drug regulatory body, Pharmacy and Poisons Board (PPB) several hospitals in the country had been given a Compassionate Use Authorisation (CUA) of Remdesivir, after studies in the US showed that it can shorten the recovery period of Covid-19 patients.
As a result, Kenya approved Remdesivir, a drug originally developed to treat Ebola. It is one of the two drugs that can work against the virus. The other drug is dexamethasone.
Improves survival
Now, although it seems to be in high demand across 50 countries globally, the pharmacy Board on Sunday said it had stopped the licensing of the drug in the country after the World Health Organisation (WHO) in November issued a conditional recommendation against its use in hospitalised patients, regardless of disease severity, as there is currently no evidence that Remdesivir improves survival and other outcomes in these patients.
“The evidence suggests no important effect on mortality, need for mechanical ventilation, time to clinical improvement, and other patient-important outcomes," said the agency's guidance development group.
Following the recommendation, Pharmacy and Poisons Board’s Deputy Director, Product Evaluation and registration, Dr Peter Mbwiri Ikamati said that the drug was not issued with an emergency use authorisation, therefore it should not be in use in Kenya.
“We are not supposed to be using it because PPB did not authorise it,” Dr Ikamati told Nation.
Dr Ikamati added that the Board is currently only approving drugs whose use have been approved by the World Health Organization.
While then issuing revised guidance, WHO added, the group of experts highlighted that clinical trial data showed the drug did not increase the survival of patients.
Developing countries
It said: “The panel interpreted the evidence as not proving that Remdesivir is ineffective; rather, there is no evidence-based on currently available data that it does improve patient-important outcomes".
In July 2020, Kenya was among 126 low-income and lower-middle-income countries identified by Gilead Sciences to receive generic versions of the drug. It will be made by five companies licensed to produce it for developing countries.
The drug, which was trialled for Ebola but failed to work as expected, is under patent to Gilead. That means no other company in wealthy countries can produce it.
However, through a non-exclusive voluntary licensing agreement, pharmaceutical manufacturers in India and Pakistan can produce and sell the treatment at a substantially lower cost,” Gilead said in a statement.
Under the agreements, the firms have a right to technology transfer from Gilead to enable them to produce the drug quickly.
Generics are copies of brand-name drugs that have the same dosage, intended use, effects, side effects, administration routine, risks, safety and strength as the original.






