Premium
State gives nod to Covid drug despite WHO’s warning
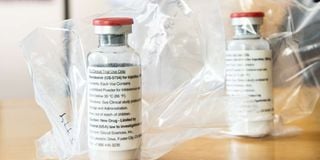
This file photograph taken on April 8, 2020, shows vials of the drug 'remdesivir' during a press conference on Covid-19 medication at The University Hospital Eppendorf (UKE) in Hamburg, northern Germany.
What you need to know:
- PPB has granted several hospitals compassionate use authorisation of Remdesivir, a drug that was approved for emergency use on Covid-19 patients two months ago.
Alfajiri Pharmaceuticals will supply to public Covid-19 treatment centres.
The government has permitted hospitals to use a medicine found to speed up recovery of severely ill Covid-19 patients despite the global health body questioning its effectiveness.
The Pharmacy and Poisons Board (PPB) has approved Remdesivir as a treatment for the disease and authorised five Kenyan companies to supply the listed hospitals with the drug.
PPB has granted several hospitals compassionate use authorisation of Remdesivir, a drug that was approved for emergency use on Covid-19 patients two months ago.
Five Kenyan companies were cleared to import the drug following the authorisation given on diverse dates beginning in June, the PPB said.
Ace Pharmaceuticals Limited was cleared to supply Mediheal Sourcing Ltd (Eldoret and Nairobi), Kisii Teaching and Referral, Karen, Mombasa, Coptic, Outspan, Nairobi South and Nanyuki Cottage hospitals.
Ripple Pharmaceuticals Ltd is to supply Pandya Memorial Hospital Mombasa while Madawa Pharmaceuticals Limited will deliver to Nairobi South and Nanyuki Cottage hospitals.
Unisel Pharma is authorised to distribute the drug to Aga Khan hospitals in Mombasa and Nairobi, Nairobi, Mombasa, Outspan, Nairobi West and Nairobi South hospitals as well as Department of Defence.
Clinical trial
Alfajiri Pharmaceuticals will supply to public Covid-19 treatment centres.
US and European drugs regulators have also since endorsed remdesivir after a clinical trial in April showed the drug shortened the recovery period of severely ill Covid-19 patients.
Remdesivir, sold under the brand name Veklury, is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein.
Remdesivir is authorised for emergency use to treat Covid‑19 in around 50 countries. Because it is given intravenously for at least five days, the drug is being used on people who require hospital admission. According to PPB, the drug is to be used as an experimental medicine by the physicians based on a risk-benefit analysis.
Compassionate use authorisation or expanded access is a potential pathway for a patient with an immediately life-threatening condition or serious disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available.
Ethics committees
“The use of the medicine is to be monitored by the ethics committees of the hospitals and monitoring reports submitted to the Pharmacy and Poisons Board,” PPB said.
But last Thursday, the World Health Organization (WHO) recommended against the use of Remdesivir in hospitalised Covid-19 patients.
“The evidence suggests no important effect on mortality, need for mechanical ventilation, time to clinical improvement, and other patient-important outcomes,” says WHO's guidance development group. While issuing revised guidance, the experts said clinical trial data showed the drug did not increase survival of patients.
“The panel interpreted the evidence as not proving that Remdesivir is ineffective, rather there is no evidence based on currently available data that it does improve patient-important outcomes.”
Data collected included results from this trial as well as three other randomised controlled trials.
“In all, data from over seven thousand patients across the four trials were considered.”
A week ago, PPB had disclosed that Square Pharmaceuticals (Bangladesh), Beximco Pharmaceuticals (Bangladesh) and Cipla Ltd (India) had applied to supply the country with Remdesivir.
Gilead Life Sciences, the manufacturer of Veklury (the innovator of Remdesivir), has also expressed interest to supply the drug through the Ministry of Foreign Affairs. It is one of the five companies licensed to manufacture the drug for developing countries. The success of the drug to manage patients has however been brought to question after some studies found it not to be effective. In October, WHO's giant solidarity trial showed that Remdesivir does not reduce mortality or the time Covid-19 patients take to recover.
The European Commission was first to give conditional approval for the use of the drug in severe Covid-19 patients following an accelerated review.
However, based on the results, the European society of intensive care medicine stopped using the drug for routine care in hospitalised Covid-19 patients. Kenya is among 126 low-income and lower-middle-income countries identified by Gilead Sciences to receive generic versions of the drug.
@emcleans [email protected]
@LeonLidigu [email protected]






