Breaking News: Former Lugari MP Cyrus Jirongo dies in a road crash
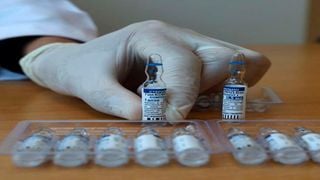
A Palestinian health worker shows vials of the Russian Sputnik V vaccine, at a vaccination centre in Gaza City, on March 27, 2021.
| Mohammed Abed | AFPNews
Premium
Questions over Russian vaccine authorisation as documents indicate high-level board clearance
Questions have emerged on who, between the Pharmacy and Poisons Board and its parent Ministry of Health, is telling the truth regarding the importation of the Russian Covid-19 vaccine, Sputnik V.
While documents seen by the Sunday Nation indicate that the board had approved the importation of the drug, top ministry officials this week said they were not aware of such authorisation. Procedurally, the board should not approve the importation of a drug into the country behind the ministry’s back.
Yet, on Monday this week at exactly 4:34 pm, a tax payment of Sh8.5 million was made to Kenya Revenue Authority, a day after 75,000 doses of Sputnik V arrived at the port of Mombasa.
The payment was made by Dinlas Pharma EPZ Limited, the importing firm, which was then instructed to get an import release note from the port. The note was processed and signed by an officer identified as Celestine on March 22.
And with that, the consignment was cleared for entry into the country. The PPB had, on March 24, announced on its official Twitter handle that it had given Emergency Use Authorisation for Sputnik V after a successful evaluation process. The PPB also announced that any company planning to import Covid-19 vaccine into the country had to sign an indemnity agreement before being cleared.
Local agents
The move was to ensure that the manufacturers or any local agents importing the vaccine take responsibility for any adverse effect on recipient patients.
With the restriction and the directive, Dinlas Pharma EPZ Limited on March 12 applied for and received a yearlong Sh201 million professional indemnity with AAR Insurance Company.
However, on Thursday last week, two Ministry of Health officials denied knowing that the vaccine was authorised for use in the country. They also said they were not aware that the vaccine was on Kenyan shelves, raising safety concerns over the planned sale of the jab to the public.
The two were head of immunisation at the ministry, Dr Collins Tabu; and Dr Willis Akhwale, the vaccine advisory task force chairman, the latter who said he was not privy to any discussions with anyone at the Health ministry on the importation of the vaccine.
In the middle of all this confusion, private health facilities are registering Kenyans for the vaccine after its arrival was made public by Russian Direct Investment Fund (RDIF) on their official Twitter handle.
Mr Nishant Mishra, a senior official at Dinlas Pharmaceuticals, said yesterday that the vaccine had been imported with the full knowledge and clearance of the PPB.
“We are using Freight in Time’s cold room to store the consignment at the required temperature levels. We have a tracker to monitor the temperature round the clock,” Mr Mishra told Sunday Nation.
When the Sunday Nation asked the PPB why the vaccines have not been distributed to private hospitals in the country, one official who sought anonymity said the clearance would be issued by the Ministry of Health.
Side effects
“Our role is limited to dealing with the safety issues and ensuring that importers meet all the requirements, after which the ministry gives distribution clearances since it is the one that deals with pricing, not the board,” the, who requested anonymity because of the sensitivity of the matter, said.
For a company to get the clearance, it has to ensure users’ safety. The distributor also needs to register users on the government-run Chanjo site, where everyone getting vaccinated has their details filed, to enable the ministry to track the number of people taking the vaccine and any side effects.
The Sunday Nation understands that Dinlas Pharma EPZ Limited on Friday trained health care workers from 27 private health facilities. It is not clear whether this was on the request of the ministry.
Sources earlier in the week told this newspaper that the vaccine will be administered by private health facilities at Sh8, 000 per dose, and yesterday Mr Mishra said Dinlas had met “all the requirements and conditions”, and that “whoever can afford to pay for the vaccine should be allowed to”.
The Sputnik V coronavirus vaccine offers around 92 per cent protection against Covid-19, according to late-stage trial results published in The Lancet.
Different vaccines have different attributes, including storage requirements, their efficacy, dosing regimens, and manufacturing platforms.
For Sputnik V, its storage temperature is -18 degrees Celsius. Its two doses are given 21 days apart, unlike the eight weeks apart for the Oxford AstraZeneca vaccine.
The AstraZeneca vaccine is already in use in the country after the delivery of 1.2 million doses under the Covax facility. Over 90,000 people have been vaccinated countrywide so far.